Stainless steel is a non-oxidizable, corrosion-resistant, rustless steel that is useful in many applications in both domestic and industrial markets. Due to its high strength, lustre, and attractive qualities, stainless steel plays a significant role in industries ranging from healthcare, food processing, and automotive.
With a plethora of areas where stainless steel finds its usage, one thing that is clear is that it not only promises shine, but is synonymous with resilience. But what is it that gives stainless steel its strength?
The answer lies in the chemistry, which makes this metal survive even in moisture-rich regions and challenging everyday life.
1. Chromium: The Protective Shield
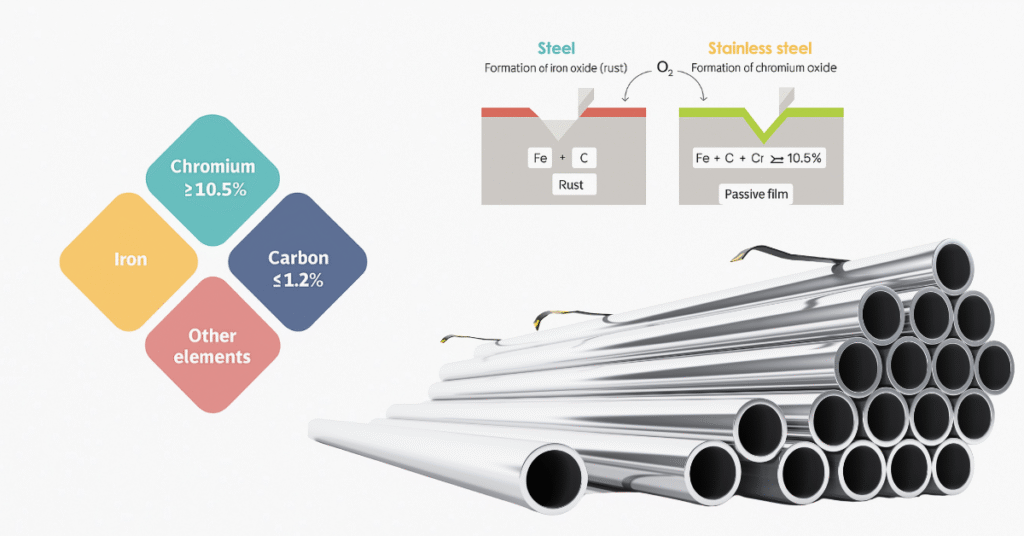
Stainless steel becomes “stainless” primarily because it contains at least 10.5% chromium, often more. When exposed to air, chromium atoms react with oxygen to form a microscopic layer of chromium oxide on the surface. This layer is only a few atoms thick, but incredibly effective. It acts like an invisible shield, preventing rust by blocking oxygen and moisture from reaching the underlying iron.
Even better: If the surface is scratched, the chromium oxide layer naturally reforms, as long as there’s oxygen in the environment. This self-healing property keeps stainless steel looking pristine and rust-free for years.
2. Beyond Chromium: Alloying Elements That Boost Performance
While chromium is the hero, other elements such as nickel, molybdenum, and nitrogen improve stability, strength, and resistance to specific corrosion types:
- Nickel enhances flexibility, toughness and shine
- Molybdenum adds durability in chloride-rich environments like seawater
- Nitrogen helps resist pitting and crevice corrosion in harsh conditions
These extras make stainless steel not just rust-resistant but also suitable for a variety of demanding industrial and domestic applications.
3. The Limits: When "Stainless" Can Fail
Stainless steel is highly resistant—but not invincible.
Risk Condition | What Happens |
Chloride-rich environment | Can cause pitting or crevice corrosion (e.g, near salt) |
Low oxygen conditions | Reduced ability to build a protective oxide layer |
Improper welding or fabrication | Disrupts the chromium-oxide layer, leading to local corrosion |
To stay corrosion-resistant, stainless steel must be handled properly during fabrication and cleaned to remove contaminants.
Why Stainless-steel Chemistry matters for Buyers & Engineers
Understanding stainless steel’s corrosion resistance isn’t just academic—it matters in how long your products last and how much maintenance they demand:
- Best suited for food processing, medical tools, marine fittings, chemical processing and architectural facades, where hygiene and durability matter.
- Requires minimal maintenance compared to other metals. Even harsh cleaning agents and acids are less effective at damaging stainless steel with a proper alloy grade.
Stainless steel is famously known as “stainless” by design, not by accident. It’s a perfect chemistry combination and smart alloy engineering gives it an ultra-thin barrier that keeps away stains, rust and decay without replacement.
If you are curious which grade of stainless steel you must purchase for your application needs, reach out to our experts at Ujala Stainless and they will guide you with the perfect alloy based on your checklist.
As a trusted distributor, stockist and supplier of stainless steel products, Ujala Stainless understands how important choosing the right grade is for your specific needs. If you’re unsure which alloy is best for your project, reach out to our experts at Ujala Stainless—we’ll help you find the perfect match that balances performance, cost and longevity.
To understand how stainless steel compares with other common types of steel, read our detailed comparison of carbon steel, stainless steel, and mild steel.

